Activated carbon is an adsorbent, which was used as early as a few hundred years BC. It was used for medical purposes and as a purifying agent. Whereas the beginnings of activated carbon's industrial applications date back to 1900,when it was used as decolourising solution in making syrup in the sugar sector. Carbons also stirred up an interest during the World War; for the first time they were being used in gas masks to protect against dangerous gases and vapours.
Activated carbon is a black, porous, amorphic sorbent. Its sorption capacity, specific surface area (reaching up to 2500 m2/g), size and distribution of pores or granularity makes activated carbon an excellent and universal adsorbent.
There are many theories concerning microscopic structure of activated carbon and its impact on the functioning of this sorbent. The basic structural unit of activated carbon is hexagonal structure of graphite, that is the form of numerous small graphite platelets. The platelets are connected with each other by chemical bonds, creating crevices, cracks and pockets, in which the pollutants are absorbed.
Availability of the internal structure of activated carbons increases the speed of the adsorption process and makes it more efficient. The most commonly used activated carbons have surface area within the limits of 800 – 1500 m2/g. The surface area of these carbons is mainly characterized by the presence of micropores with their effective diameter smaller than 2 nm. As a matter of fact, activated carbons are composed of a complicated network of pores, classified as follows:
Adsorption on the surface of activated carbons occurs mostly in micropores and in small parts in mezopores, while the macropores only function as channels through which the adsorbate flows into mesopores and on the surface of micropores. The distribution of pore sizes in a given carbon depends on the type of raw material used as well as on the methods and conditions of its production.
Purification using activated carbon is made possible by exploiting the phenomenon of adsorption. Simply put, adsorption involves the accumulation of gas or liquid molecules on the surface of a solid material known as the adsorbent.
Adsorption can be categorized by the type of interactions involved:
The type of adsorption can be identified based on:
Heat of the process – low for physical adsorption, but significantly higher for chemisorption reactions.
Reversibility of the process - substances adsorbed via physical interactions can be easily removed during regeneration, whereas chemically adsorbed layers are difficult to remove and require harsh conditions.
Thickness of the adsorption layers – physical adsorption layers may correspond to several molecular diameters of the adsorbate (depending on pressure and temperature), while chemisorption typically forms monolayer adsorption.
Due to its high purity and ease of use, activated carbon is a widely applied adsorbent. Since adsorption does not change the chemical composition of the purified medium, activated carbon’s properties are utilized in many technological processes.
Specialized knowledge of adsorption processes combined with the extensive experience of the engineering team at ACES Sp. z o.o. enables us to select the most suitable type of activated carbon to fully meet our clients’ purification requirements.
Which compounds can be adsorbed by activated carbon?
Activated carbon adsorbs organic compounds as well as some larger inorganic molecules such as iodine or mercury. Approximately 90% of organic compounds can be effectively adsorbed by activated carbon, while about 10% are considered weakly or non-adsorbable. However, adsorption effectiveness varies depending on many factors.
Key factors include:
Applying these principles along with product-specific knowledge is crucial when selecting an activated carbon for filters. The ACES Sp. z o.o. team leverages its experience to ensure the proposed carbon filters for gas purification deliver maximum adsorption performance. For further questions, please contact us.
Activated carbon is produced from carbonaceous raw materials such as coal, coconut shells, wood, or peat.
The production involves two main stages:
The method and conditions of activation significantly influence the physical and chemical properties of the final formed activated carbon product, including pore volume, surface area, and surface functional groups.
Common raw materials for activated carbon production include:
About 60% of all activated carbons used worldwide are derived from bituminous coal. The final characteristics of activated carbon depend on numerous factors during production. Bituminous coals are known for easily developing a highly porous structure; lignite exhibits the largest pore volume; coconut shell carbons are distinguished by a high number of active sites, hardness, and abrasion resistance; anthracite contains the highest elemental carbon content, making it the hardest and most resistant to aggressive environments. Wood-based carbons are used as powdered carbons with low bulk density and a proportionally large number of macropores.
Thus, the origin of the activated carbon determines its porosity and suitability for various industrial processes.
|
Raw Material |
Porous Structure |
Application |
Example Use |
|
Bituminous coal |
High porosity, predominance of mesopores |
Liquid phase adsorption |
Water treatment, wastewater purification, soil remediation |
|
Lignite |
Low porosity, predominance of macropores |
Liquid phase adsorption |
Product decolorization |
|
Coconut shells |
Very high porosity, predominance of micropores |
Liquid and gas phase adsorption |
Water treatment, residual chlorine and ozone removal, VOC adsorption |
|
Anthracite |
Very low porosity, predominance of mesopores |
Gas phase adsorption |
VOC adsorption |
|
Wood |
Very high porosity, predominance of macropores |
Liquid phase adsorption |
Water treatment with powdered carbon, product decolorization |
Structural characteristics of activated carbons depending on their origin
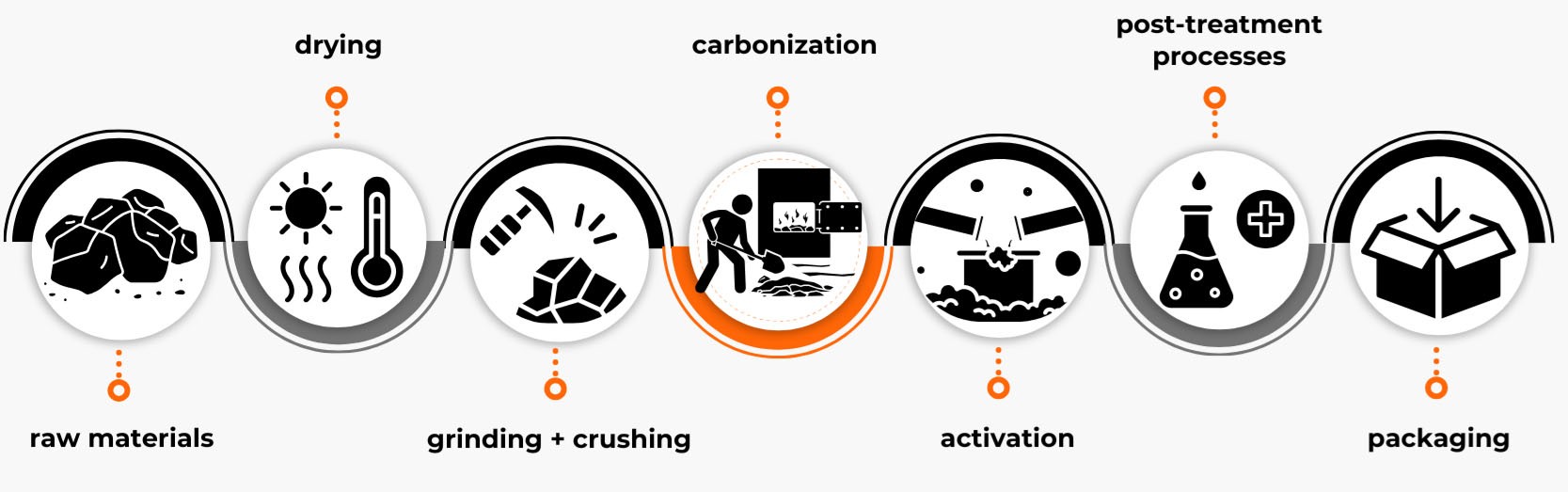
Selection of raw material:
The process begins with the careful selection and sourcing of the appropriate feedstock. Depending on the intended application of the final product, raw materials may include bituminous coal, lignite, coconut shells, wood, or anthracite.
Drying:
Natural raw materials such as wood typically contain a high moisture content. Therefore, the first processing stage involves drying the material to reduce water content, enhancing the efficiency of subsequent steps.
Grinding, crushing, agglomeration, and shaping:
Based on the initial particle size and final product requirements, the raw material may undergo preliminary grinding to achieve the desired fraction. If the product is intended to be shaped (e.g., pellets, cylinders), the material is then agglomerated or formed into the required shape.
Carbonization:
This is the first essential stage of activated carbon production. The material is subjected to heat treatment in an oxygen-free atmosphere at 300–400°C for approximately 12 hours. During this process, up to two-thirds of volatile substances are removed, and the initial pore structure begins to develop. The resulting intermediate product, known as char, is then cooled for an additional 12 hours.
Activation:
The char undergoes activation, the most critical step in the production process, aimed at developing a porous structure:
Once the activation process is complete, the activated carbon is cooled, dried, and screened to achieve the required granulation. For powdered grades, additional milling is performed.
Post-treatment processes:
Depending on the application, activated carbon may undergo further treatment:
Packaging and quality control:
Samples are sent to accredited laboratories for quality control testing, where key parameters are thoroughly analyzed. Once the product meets all quality specifications, it is repackaged and prepared for distribution.